4. 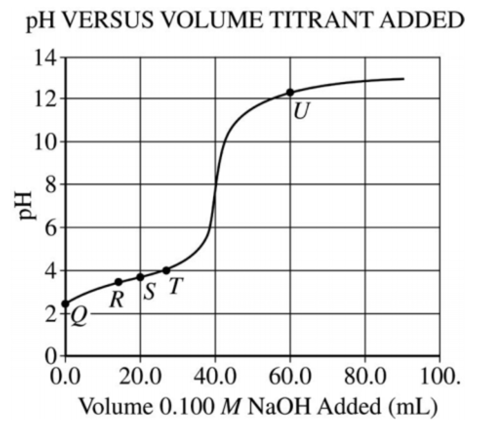
A 50.0 mL sample of an acid, HA, of unknown molarity is titrated,and the pH of the Resulting solution is measured with a pH meter and graphed as a function of the volume Of 0.100 M NaOH added.
At point R in the titration,which of the following species has the highest concentration?